Getting older is the biggest known risk factor for AD. According to the National Institute on Aging, the number of people who have AD doubles every five years after age 65 and about a third of those 85 and older could have it.
Studies of beta-amyloid and tau are ongoing in labs at Pitt and across the world to better understand normal and abnormal aging. Researchers are examining other brain changes, including atrophy or shrinkage, and inflammation.
In the Marmo-AD project, Pitt researchers are breeding a colony of non-human primates called marmosets to have genetic risk factors for AD. They will carefully monitor them throughout their lifespans to watch for preclinical signs of the disease, identify predictive factors, and possibly test interventions.
Women have a higher risk of developing AD than men, which until recently was thought to be related to their longer lifespans. Now, scientists are examining the relationship between the hormonal changes of perimenopause, hot flashes and the cardiovascular system, and changes in the brain that might contribute to AD.
According to the National Institutes of Health, scientists have now identified more than 70 gene regions associated with Alzheimer’s disease. Genetic testing is not routinely available to predict the likelihood of eventually developing AD.
APOE (“ay-poh ee”): This gene has several variants and makes a protein involved in cholesterol transport. Each person gets one copy, or allele, from each parent. APOE 3 is the most common variant and isn’t linked to either an increased or decreased susceptibility for AD. APOE 2 is the least common and may confer some protection against the disease. APOE 4 increases the risk of developing AD: 15-25 percent of the population inherited one copy of it from a parent, and 2-5 percent have two copies, one from each parent. A person who has two copies (“homozygous”) of APOE4 has an 8- to 12-fold increased risk of getting AD.
APP: Changes in the Amyloid Precursor Protein (APP) gene have been linked to increased risk of early-onset Alzheimer’s. The gene makes amyloid precursor protein, which is normally cleaved into soluble Amyloid Precursor protein (sAPP) and beta-amyloid. In Alzheimer’s, gene changes lead to overproduction of beta-amyloid, which forms clumps in the brain. The gene for APP lives on chromosome 21; people who accidentally get three copies of the gene during embryonic development, which causes Down Syndrome, also can make too much beta-amyloid. About a third of people who have Down Syndrome have AD in their 50s; about half have it in their 60s.
PSEN1: The presenilin 1 (PSEN1) gene on chromosome 14 makes a component of the enzyme gamma secretase, which cleaves APP into sAPP and beta-amyloid. About 70 percent of early-onset AD cases are caused by mutations in the PSEN1 gene.
PSEN2: The presenilin 2 (PSEN2) gene on chromosome 1 also makes proteins involved in APP processing. About 5 percent of early-onset AD are due to mutations in this gene.
There are associations between AD risk and cardiovascular factors, such as high blood pressure, diabetes, and smoking. These conditions can lead to inflammation and other changes in the brain’s blood vessels that might contribute to the development of cognitive impairment.
Pitt researchers have established a Brain Bank containing thousands of cell and tissue samples donated by AD patients to help make scientific advances for the benefit of future generations.
In addition, Pitt’s Alzheimer’s Disease Research Center (ADRC) is home to multiple clinical trials of experimental interventions aimed at slowing or stopping the disease, as well as studies to help family caregivers.
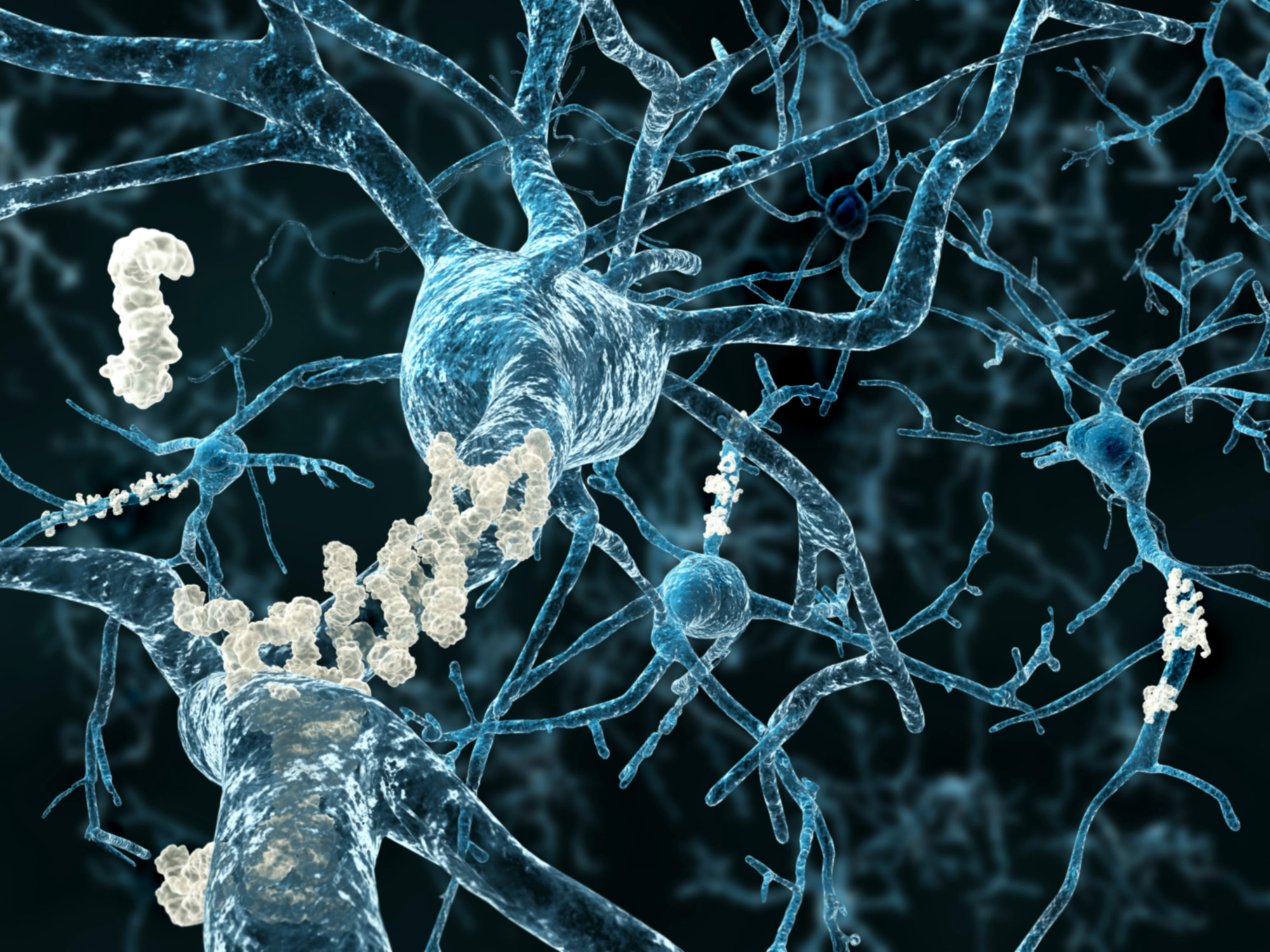
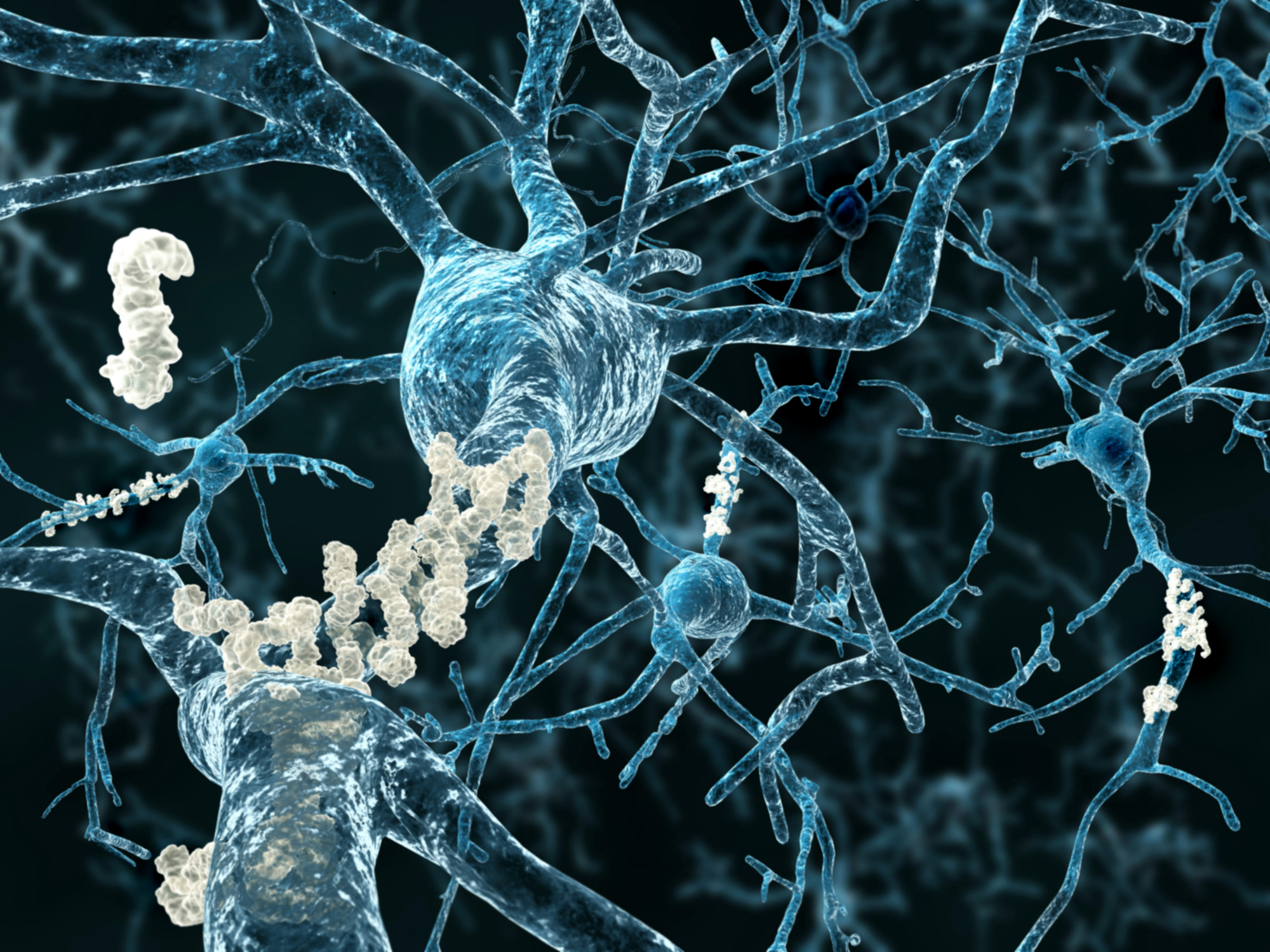
Alzheimer’s disease (AD) is the most common form of dementia and the 7th leading cause of death in the United States. Over many years, a protein called beta amyloid forms clumps or plaques in the brain, a hallmark of the disease. Another protein called tau changes also, tangling into fibers that also deposit in the brain. These abnormalities destroy neurons, shrinking the brain and leading to severe memory loss, confusion, mood and behavior changes, and more.
The disease was named for Dr. Alois Alzheimer, who in 1901 assessed a 50-year-old woman who was forgetful, confused, and aggressive. Upon her death in 1906, the pathologist examined her brain and found it to be riddled with what would eventually be called beta amyloid plaques and tau tangles.
It might seem that amyloid and tau abnormalities are the cause of Alzheimer’s, but scientists now have reason to suspect that there are biological events – still unknown – that trigger those protein changes. For many years, researchers worked from the premise that reducing amyloid plaque burden in the brain could relieve symptoms – an approach known as the “amyloid hypothesis.”
However, hundreds of trials of drugs aimed at reducing brain amyloid burden have failed to make an impact on symptoms. Recently, three amyloid-targeting drugs (aducanumab, lacanemab, and donanemab) demonstrated in clinical trials a very modest effect on slowing early-stage cognitive decline. They do not cure the disease, nor do they restore memory, cognition, or other Alzheimer’s-induced brain changes.
Both amyloid plaques and tau tangles can be found in the brains of people who didn’t have AD when they died, suggesting those changes might also be part of normal aging.